Description
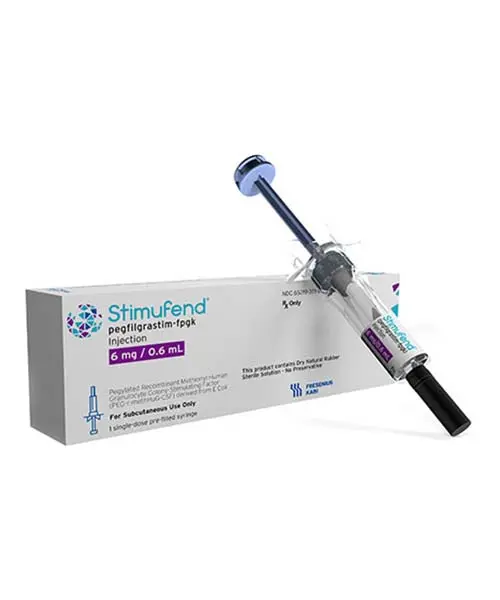
Approved accessible “pegfilgrastim-fpgk injection”
Pegfilgrastim is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in people with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia; and to increase survival in people acutely exposed to myelosuppressive doses of radiation (hematopoietic subsyndrome of acute radiation syndrome)
Pegfilgrastim, sold under the brand name Neulasta among others, is a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. It serves to stimulate the production of white blood cells (neutrophils). Pegfilgrastim was approved for medical use in the United States in January 2002, in the European Union in August 2002, and in Australia in September 2002. Stimufend was approved by the US Food and Drug Administration in September 2022.
How can 1 go about obtaining pegfilgrastim-fpgk injection?
If pegfilgrastim-fpgk injection are not yet approved or available in your area, you may request its price in India under a specific brand name through IPN. For assistance and guidance, you can contact us by providing complete details. Our pharmaceutical expert will help you find a suitable and practical solution to your query. To receive comprehensive support, please call us at +91 9891 296 838 (Mr. Tarun) / +91 9811 747 774 (Mr. Neeraj).