Description
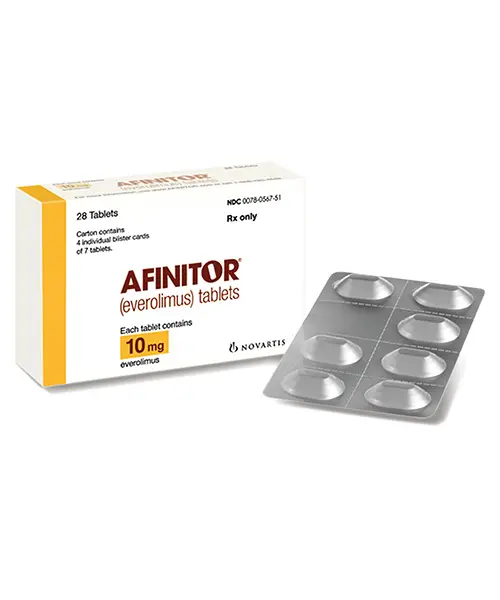
Approved accessible “everolimus tablets”
AFINITOR is a kinase inhibitor indicated for the treatment of:
• postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane after failure of treatment with letrozole or anastrozole.
• adults with progressive neuroendocrine tumors of pancreatic origin (PNET) that is unresectable, locally advanced or metastatic. The safety and effectiveness of AFINITOR in the treatment of patients with carcinoid
tumors have not been established.
• adults with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib.
• adults with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery. The effectiveness of AFINITOR in treatment of renal angiomyolipoma is based on an analysis of durable
objective responses in patients treated for a median of 8.3 months. Further follow-up of patients is required to determine long-term outcomes.
• adults and children ≥ 3 years of age with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis (TSC) who require therapeutic intervention but are not candidates for curative surgical
resection. The effectiveness of AFINITOR is based on an analysis of change in SEGA volume. Clinical benefit such as improvement in diseaserelated symptoms or increase in overall survival has not been demonstrated.
How To access everolimus tablets?
Afinitor (everolimus tablets) for oral suspension Initial U.S. Approval: 2009
To access everolimus tablets sold under the brand name can be made available on request to patient if the drug has not been approved or is not available in your country. For information or advice, call IPN’s Pharmaceutical Expert. Our expert will be happy to take your query and guide with an effective solution. Be free and call today for full support at +91 9891 296 838 (Mr. Tarun) / +91 9811 747 774 (Mr. Neeraj)